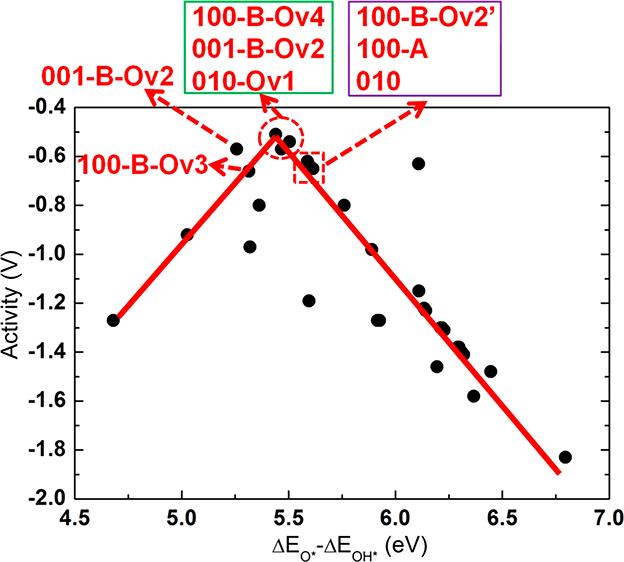
Neutral oxygen vacancies (Ov’s) in semiconductor oxides give rise to excess electrons that have the potential to affect the binding of adsorbates to the surface through surface-to-adsorbate charge transfer, and, as a result, to alter the over-potential (OP) of reactions on oxygen-deficient materials compared to stoichiometric materials. We report a systematic computational investigation of the effects of Ov’s on the Oxygen Evolution Reaction (OER) over-potential for β-Ga2O3, a d10 semiconductor that has been shown to exhibit high activity for water splitting. We investigated eighteen β-Ga2O3 surfaces / slabs, with and without Ov’s and observed a clear dependence of OER activity on Ov’s. A general finding emerged, that the excess electrons associated with Ov’s are found to participate in charge transfer to OER intermediates, making their bonds to the surface more ionic and stronger, depending on the amount of charge transfer. The OER reaction step free energies are significantly affected and the ensuing over-potentials are altered. The amount of charge transfer varies with the types of intermediates (OH*, dangling O*, surface-bound peroxo O*, and dangling OOH*), their open valencies, and their electronegativity. The work function and the position of the gap states of the excess electrons in the band gap are found to be useful descriptors of whether and how much Ov-induced charge transfer may occur and affect the over-potential. However, it was also found that the chemical environment of the O atom where the vacancy was created, may have a negating effect on the general observation. Specifically some Ov structures underwent a strong relaxation to form Ga-Ga bonds, trapping the vacancy electrons, and preventing them to engage in charge transfer. Oxygen vacancies are common defects in photocatalyst materials so that our investigation can provide guiding principles for designing efficient photocatalysts. This paper was published on Chemistry of Materials, 2018, 30, 7714-7726.