Helicene chemistry has over 100 years history, thiahelicenes, as a kind of typical heterohelicenes, have been intensively studied. Selenophene is a close analogue of thiophene. In general, substitution of sulfur with selenium leads to many differences. However, limited research has been accorded to selenophene derivatives than thiophene derivatives, because the preparation of these derivatives remains high challenge. Recently, two kinds of selenophene-based hetero[7]helicenes have been synthesized, and intermolecular interactions such as C-Se, C-S, and Se-Se were observed in the crystal packings. The study results have been published in J. Org. Chem. (IF = 4.85).
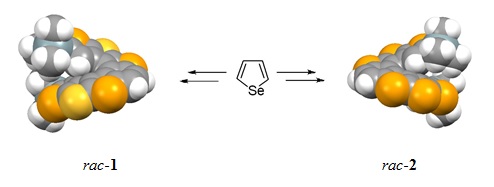
Starting from selenophene, through bromination, TMS protection, bromine dance reaction, Li/Br exchange CuCl2 coupling, deprotonation intramolecular cyclization with selenium and bis(phenylsulfonyl)sulfide five steps reactions, two new kinds of selenophene-based building blocks, namely, 2,5-di(trimethylsilanyl)-diseleno[2,3-b:3',2'-d]selenophene (thiophene) ((TMS)2-DSS, (TMS)2-DST) were obtained. The two new building blocks can be used not only as building blocks for selenophene-based helicenes but also for preparation of new types of organic functional materials based on selenophene. Starting from (TMS)2-DSS and (TMS)2-DST, via four steps: bromination, formylation, McMurry reaction, and photocyclization, selenophene-based hetero[7]helicenes, namely, rac-1and rac-2 were obtained, and the total yields are 6.5% and 6.1%, respectively. Single-crystal analysis shows the predicted two-fold symmetry for rac-1 and rac-2 and the existence of multiple short contacts, including C-Se, C-S, and Se-Se interactions.
After the thiophene helicenes, double helicenes and triple helicenes, selenophene-based hetero[7]helicenes rac-1 and rac-2 are our research group synthesized new kinds of helicene compounds, namely selenophene helicenes. The current work provides a foundation for the development of selenophene chemistry.